There are four major projects in the laboratory:
1. Treatment of Sleep Disordered Breathing by Targeting Leptin Signaling
Obesity is the most common risk factor for sleep-disordered breathing (SDB). The most common form of SDB, obstructive sleep apnea (OSA), affects 50% of obese patients. OSA is recurrent upper airway obstruction during sleep caused by a loss of upper airway muscle tone. Obesity hypoventilation syndrome (OHS) is a life-threatening severe form of SDB characterized by daytime hypercapnia and hypoventilation during sleep. OHS is observed in 10-20% of obese patients with OSA. OHS is driven by decreased CO2 sensitivity and depressed ventilatory responses to CO2. Treatment of upper airway obstruction during sleep in OHS patients with continuous positive airway pressure (CPAP) restores CO2 sensitivity and treats OHS. However, poor adherence to CPAP limits its therapeutic use. There is no effective pharmacotherapy for OHS.
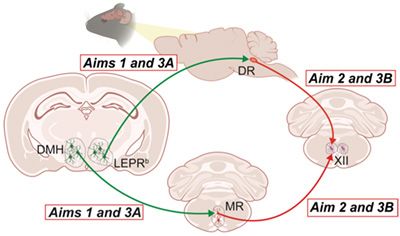
Our efforts are focused on leptin, an adipose-produced hormone, which suppresses food intake and increases metabolic rate. In leptin deficient obese ob/ob mice, leptin also augments hypercapnic sensitivity and improves upper airway patency during sleep. However, human obesity is characterized by high levels of circulating leptin and resistance to metabolic and respiratory effects of systemic leptin. Leptin resistance may be mediated by multiple mechanisms including limited permeability of the blood-brain barrier and impaired signaling via the leptin receptor LEPRb. We have shown that diet-induced obese (DIO) mice emulate all features of OHS, including awake hypercapnia, upper airway obstruction during sleep, sleep hypoventilation, high plasma leptin levels and leptin resistance. We have also shown that resistance to intravenous and intraperitoneal leptin treatment in DIO mice can be overcome by intranasal leptin administration. We identified LEPRb+ neurons in the dorsomedial hypothalamus (DMH) as a likely target for the respiratory responses to leptin. We have linked the effects of leptin to activation of the hypoglossal motoneurons (XII MN), which dilate the pharynx by stimulating the main tongue protrudor, genioglossus. However, mechanisms, and a neuronal network with potential co-activators, by which leptin stimulates XII MN, relieves pharyngeal obstruction during sleep and treats SDB remain unknown. Based on our previous work and Preliminary Data, we propose that, in obesity, therapeutic effect of leptin on upper airway and ventilation during sleep is mediated by LEPRb+ neurons in DMH and requires activation of downstream serotonergic (5-hydroxytryptamine, 5-HT) neurons, that, in turn, activate XII MN (Figure 1).
2. Melanocortin 4 Receptor Agonists to Treat Sleep Disordered Breathing in Obesity
Human and mouse DIO become resistant to leptin limiting leptin as a pharmacotherapy. This proposal is focused on identifying pharmacological targets downstream of the leptin network to treat OHS.
Leptin upregulates pro-opiomelanocortin (POMC), which is a pre-hormone post-transcriptionally processed into several peptides, including α-melanocyte stimulating hormone (α-MSH), a ligand for the melanocortin 4 receptor (MC4R) promoting energy expenditure. MC4R agonists increase energy expenditure and suppress food intake in obese rodents and humans. MC4R mutations have been associated with 1-6% of human obesity. In addition, leptin inhibits agouti-related peptide (AgRP) neurons of the hypothalamus, and AgRP is an MC4R blocker. The MC4R agonist setmelanotide has been approved for treatment of genetic forms of obesity linked to mutations in the POMC/MC4R/leptin pathways. We have previously shown that agouti mice overproducing AgRP have attenuated HCVR. An MC4R blocker SHU9119 suppressed the HCVR at baseline and after leptin administration. Obese children with MC4R mutations develop severe SDB. However, the role of MC4R in control of breathing remains unknown.
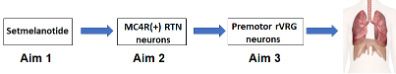
Our exciting preliminary data shows that (1) setmelanotide robustly augments the HCVR and treats SDB in DIO mice; (2) Mc4r mRNA is abundantly expressed in Phox2b positive neurons in ventilatory control centers of the medulla, including a parafacial region containing the retrotrapezoid nucleus (RTN); (3) chemogenetic activation of MC4R (+) neurons in the parafacial region increases baseline ventilation and HCVR without any effect on metabolism; (4) MC4R (+) parafacial neurons project to brainstem respiratory premotor neurons, that, in turn, project to the phrenic motor nucleus. Our overarching hypothesis is that MC4R agonists treat OHS by augmenting hypercapnic sensitivity in MC4R+ neurons of the parafacial region, which is a major center of CO2 sensitivity. We will address our hypothesis in three aims (Figure 2)
3. Leptin Signaling in the Carotid Body: Mechanisms and Consequences
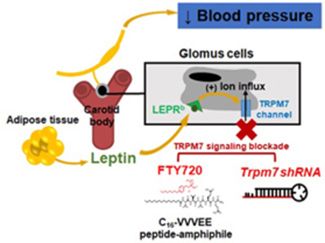
The CB are major peripheral sensors of hypoxia, metabolic, and cardiovascular homeostasis transmitting input via the carotid sinus nerve to the medullary centers, which results in the activation of the sympathetic nervous system. CB play a role in the pathogenesis of hypertension. Leptin infusion induces hypertension in obesity acting via the TRPM7 (transient receptor potential cation channel subfamily M member 7) cation channel in the carotid bodies. A TRPM7 blocker FTY720 (Trpm7 inhibitor) and Trpm7 gene silencing using short hairpin RNA (shRNA) applied to CB abolished leptin-induced elevations in blood pressure.
4. Melanocortin 4 Receptor Agonists in Opioid-Induced Respiratory Depression
Opioid use disorder is a national crisis that affects public health, as well as social and economic welfare. The opioid crisis has been greatly exacerbated by the increased availability of synthetic opioids, such as fentanyl, and by the increased prescribing of opioid pain relievers. The primary cause of death associated with opioids is opioid-induced respiratory depression (OIRD). Naloxone is a competitive antagonist of MORs and other opioid receptors, has a rapid onset and highly effective in acutely reversing OIRD, but re-dosing is necessary to reverse long-acting synthetic opioids. A recently approved MOR antagonist, nalmefene, is long-acting. However, both naloxone and nalmefene induce acute withdrawal and reverse analgesia. Our approach targets FDA-approved agents that have been shown to stimulate breathing in sleep disorders and repurpose them for the treatment of OIRD. We have shown that leptin stimulates breathing by increasing the hypercapnic ventilatory response and treats hypoventilation in obese animals. We have also shown that leptin prevents OIRD and decreases opioid mortality in a mouse model. However, leptin resistance often limits leptin as a pharmacotherapy. Leptin upregulates pro-opiomelanocortin, which is a pre-hormone post-transcriptionally processed into several peptides, including α-melanocyte stimulating hormone (α-MSH), a ligand for the melanocortin 4 receptor (MC4R). The MC4R agonist setmelanotide (SET) has been approved by the FDA for treatment of genetic forms of obesity. Our exciting preliminary data in mice shows that SET effectively treats both sleep disordered breathing (SDB) and OIRD by increasing respiratory rate and inducing a greater than 3-fold decrease in the number of apneas without affecting analgesia, and that the likely mechanism of MC4R agonists is via activation of brainstem respiratory neurons.